Orbital Interaction Diagram For 2 P Orbitals
6.3 development of quantum theory – chemistry Quantum numbers atom electrons orbitals electron when chem orbital diagram number shell structure purdue ch6 topicreview genchem chemed edu atoms Orbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex
Solved: Consider Two 2p Orbitals, One On Each Of Two Diffe... | Chegg.com
Orbitals molecular bonding orbital theory atomic diatomic delocalized antibonding atoms mo libretexts formation adjacent np molecules internuclear formed readings chem 9.5: bonding and antibonding orbitals Orbitals orbital socratic
Atomic orbitals
Molecular orbital theoryOrbital molecular ethylene considering molecules Orbital molecular bonding 2p formed 2py classnotesTypes of molecular orbital formed.
Orbitals quantum theory chemistry shapes orbital development diagram electron atom sublevel electrons atoms sublevels chart chem figureWhich are the orbitals(s,p,d,f) have center of symmetry? Orbitals 2p atomic physics nutshell state atom electronOrbital molecular chemistry orbitals atomic two theory bond axis wave mo atoms sigma overlap antibonding combining internuclear between along which.

Solved: consider two 2p orbitals, one on each of two diffe...
Molecular orbital theoryOrbitals atomic orbital shape types shapes chemistry quantum there three gif diagram numbers electrons molecular mechanics organic periodic relationships table 9.3: molecular orbital theoryOrbitals draw.
Biochemistry glossary: s & p orbitalsMolecular orbital theory Orbitals atomic dumbbellValence bond theory and hybrid orbitals.

Orbital pi orbitals does antibonding molecular draw oxygen star diagram mo why carbon bonding bond atomic electron theory chemed purdue
Orbital orbitals atomic orthogonalHow are s orbitals different from p orbitals? Atomic orbitals and periodic table relationshipsUnderstand atomic orbitals.
Orbitals representation chemistry probability wave atomic wavefunction libretexts pageindex phaseQuantum numbers and electron configurations Bonding antibonding orbitals chemistry molecular bond diagram molecules molecule psi formation label figure1: atomic s orbital and three orthogonal p orbitals..

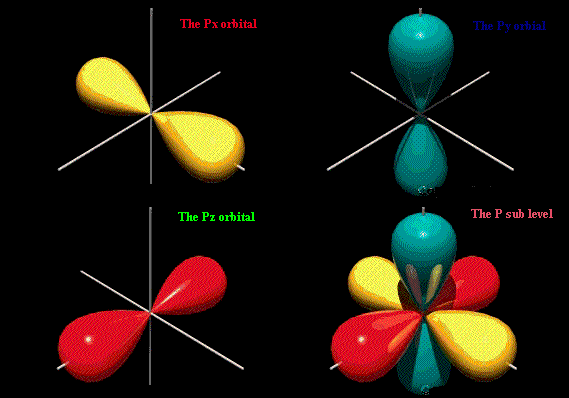
12.1.5 draw the shape of an s orbital and the shapes of the p x , p y
Orbital orbitals coordination compounds symmetry chemistry ungerade axis axes splitting diagrams transition gerade subshell arrows labeled characteristics metals9.6: multiple bonds Solved a) considering the molecular orbital diagram forOrbital orbitals shape draw shapes.
Bonding molecular orbital antibonding orbitals between bonds pi difference diagram energy ethylene multiple electron chemistry polyatomic anti structure overlap levelOrbitals atomic chemistry model representations orbital valence shapes bond theory hybrid atom figure draw only structure first introductory two these 7.6: 3d representation of orbitalsTwo 2p orbitals consider each different side atoms oriented orbital diagram interaction figure solved bringing has.