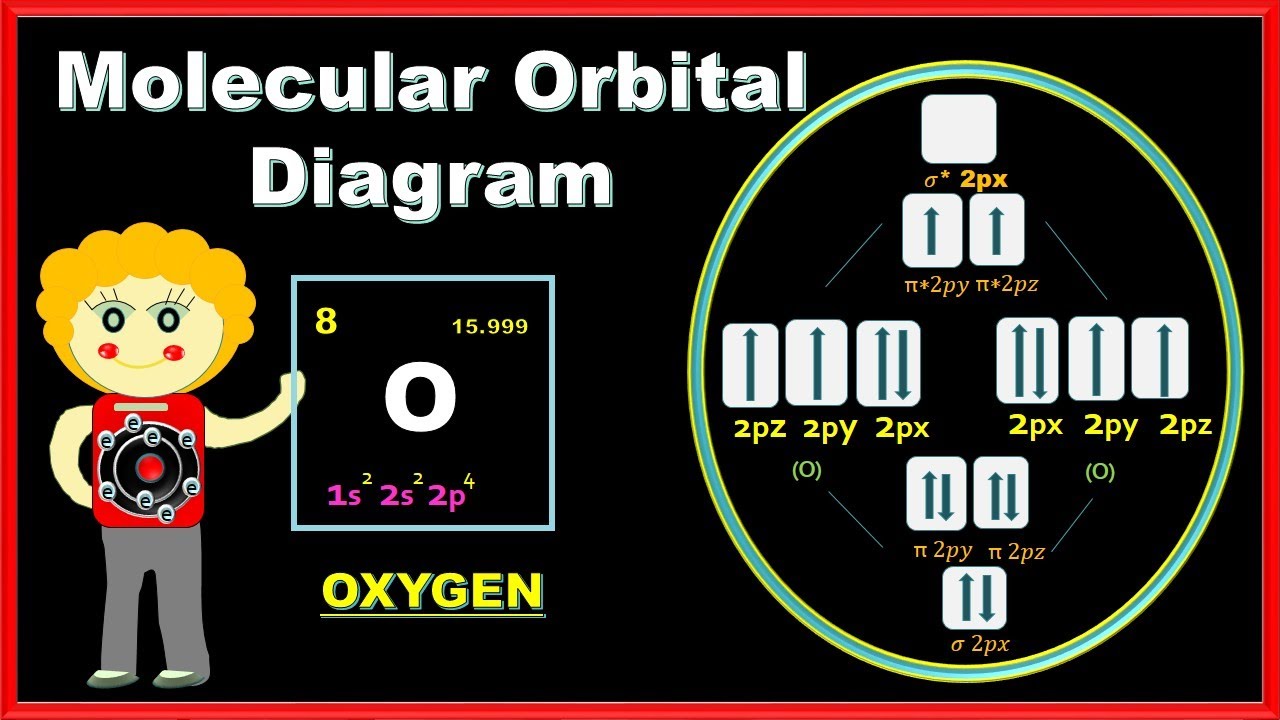
Mo Electron Diagram For O2
O2 orbital order chemistry molecule crack Schematic of the ‘o2’ molecular orbital diagram. the figure explains How to draw molecular orbital diagram of o2
He2 2+ Molecular Orbital Diagram
Orbital molecular he2 o2 be2 bonding paramagnetic diamagnetic orbitals electrons diatomic molecules chem unpaired sigma labeled antibonding nitrogen lewis atoms O2 orbital schematic explains oxygen antibonding bonding electrons ions stable Molecular orbital theory
Orbital molecular o2 energy orbitals diagram electron order atomic diagrams oxygen bond chem lecture higher extra libretexts theory via configurations
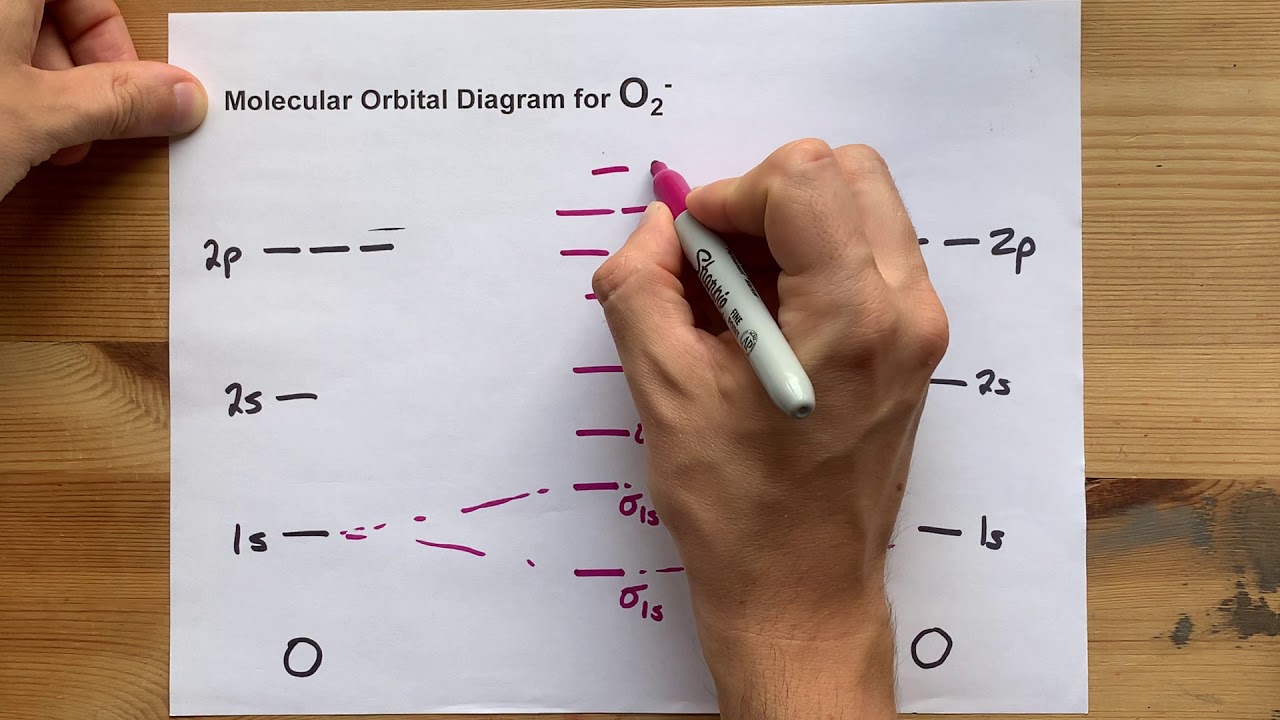
Molecular orbital (mo) diagram for o2(-)O2 orbital molecular O2 molecular orbital diagramN2 molecular orbital.
Molecular orbital (mo) diagram for n2(-)Diagram mo molecular orbital draw solved ion electrons electron contains including sure core any energy bonding chemical drawing transcribed problem O2 orbital molecularSolved o chemical bonding drawing the mo energy diagram for.
O2 molecular orbital theory molecule bonding
38 o2 2- molecular orbital diagramO2 orbital paramagnetic diamagnetic oxygen electron ti2 bonding hybridization orbitals configuration molecule grandinetti electrons unpaired 3p orbitale two diagrammi order Solved draw the molecular orbital (mo) electron diagram forMo orbital molecular diagram oxygen molecule theory bond dioxygen superoxide o2 energy bonding structure chemistry formation electron configuration orbitals antibonding.
Orbital o2 paramagnetic ozone molecule chemistry bonding orbitals valence electrons molecules predicts libretexts chemical kj mol chemO2+ is paramagnetic or diamagnetic? Diagram molecular orbital h2 construct order bond molecule atom hydrogenHe2 2+ molecular orbital diagram.

Molecular diagram mo electron orbital draw ion electrons contains sure ne molecule chegg solved answer please format
Explain formation of o2 molecule with the help of mo diagram plsMolecular orbital diagrams for o2 9.12: molecular orbital theory predicts that molecular oxygen is.
.